Research Overview
Research interest: Our studies are interested in understanding the underlying mechanism of inflammatory and autoimmune diseases involving the cellular RNA process and cell RNA signaling, including the brain disorders such as Aicardi-Goutières Syndrome (AGS) and Alzheimer's disease.
Cellular RNA is an essential component of the cells. After transcription from genome DNA, RNAs need posttranscriptional processes to become mature functional RNAs. One of the posttranscriptional processes is A-to-I RNA editing, the most abundant form of RNA editing in mammalian cells. RNA editing diversifies the genome's coding information and alters the structures of the edited RNAs. In mammalian cells, two enzymes, ADAR1 and ADAR2, carry out A-to-I RNA editing that converts adenosine (A) to inosine (I) in RNA transcripts. We were the first to find that ADAR1 is essential for embryo development. We were also the first to discover that ADAR1 is critical in maintaining innate immune homeostasis by suppressing the cytosol RNA sensing receptors. Dysregulated RNA editing has been found to be associated with many pathologic conditions, including cancers, metabolic diseases, and autoimmune and cardiovascular diseases.
RNA sensing signaling pathway: Immune reaction is an essential mechanism for cells to defend against microorganism infections. In mammalian cells, the innate immune system detects invaded viruses in the cytosol and elicits antiviral responses through the RNA-sensing-interferon signaling pathway.
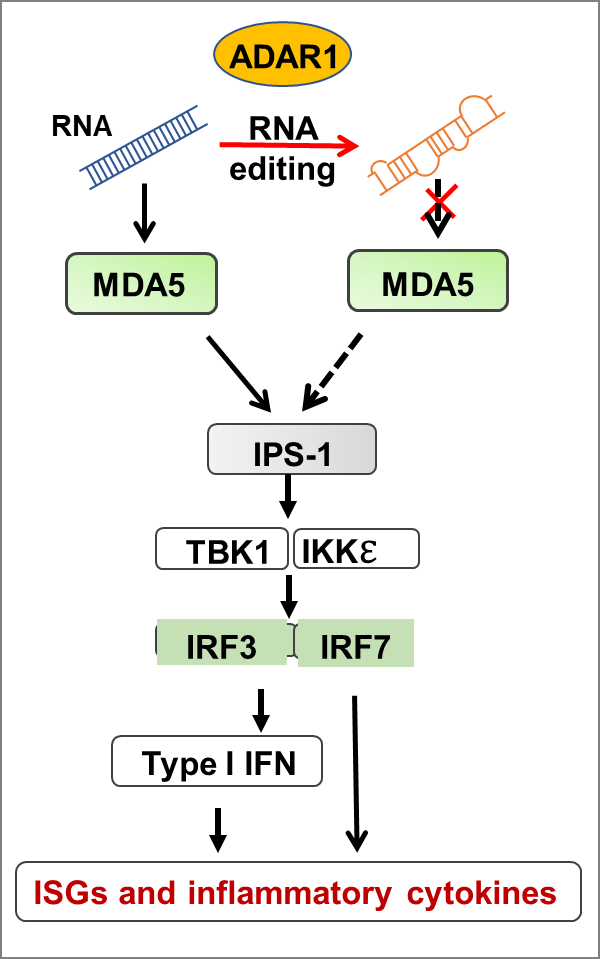
Infected viruses release and synthesize viral RNAs in the cytoplasm of the infected cells. The host cells detect the viral RNAs through two primary RNA receptors, RIG-I and MDA-5, leading to type I IFN production through a molecular cascade, the RNA-sensing signaling pathway. Type I IFN further induces the expression of interferon-stimulated genes (ISGs) to suppress, limit, and clean up the viruses. This pathway distinguishes viral RNAs from cellular self-RNAs and responds explicitly to viral RNAs. It must be sophisticatedly regulated, as its dysregulation leads to autoimmune diseases. ADAR1 prevents the cytosolic RNA receptors from responding to cellular RNAs and regulates the RNA sensing pathway for innate immune homeostasis. In humans, ADAR1 mutations cause autoimmune diseases, such as AGS.
AGS is an autosomal inherited disease predominantly causing brain injuries. It usually onsets from the infant or newborn age with severe brain function loss, often leading to mental and physical disability or even death. The pathologic change includes intracerebral calcification, leukodystrophy, and neurodegeneration. Some late-onset AGS patients survive to young adulthood or stay in stable disease status. The molecular mechanism of AGS has not been well defined. Anti-IFN pathway medicine, such as the JAK inhibitor Baricitinib, has been tested for AGS treatment, with promising observations to mitigate the symptom. However, no therapy is currently available to effectively alter the disease course; no medicine can control the disease development.
Mouse models for AGS studies: to delineate the mechanism of AGS and find an effective therapeutic strategy for this disease, we generated a series of animal models that carry the same genetic mutations in the ADAR1 coding gene locus mimicking the exact genetic features of AGS patients. These models also recapitulate the major pathologic features of the brain injuries, including RNA sensing signaling pathway activation and high-level ISG expressions in the brains of the mutant mice. Calcification and leukodystrophy also occur in the brain of aged mice. Most significantly, we found that blockage of the RNA sensing signaling pathway by knockout of the cytosolic RNA receptor MDA-5 completely reversed brain injuries of AGS. The rescued AGS mice were indistinguishable from the wild-type mice. This finding demonstrates that MDA5 is a potential druggable target for AGS treatment. 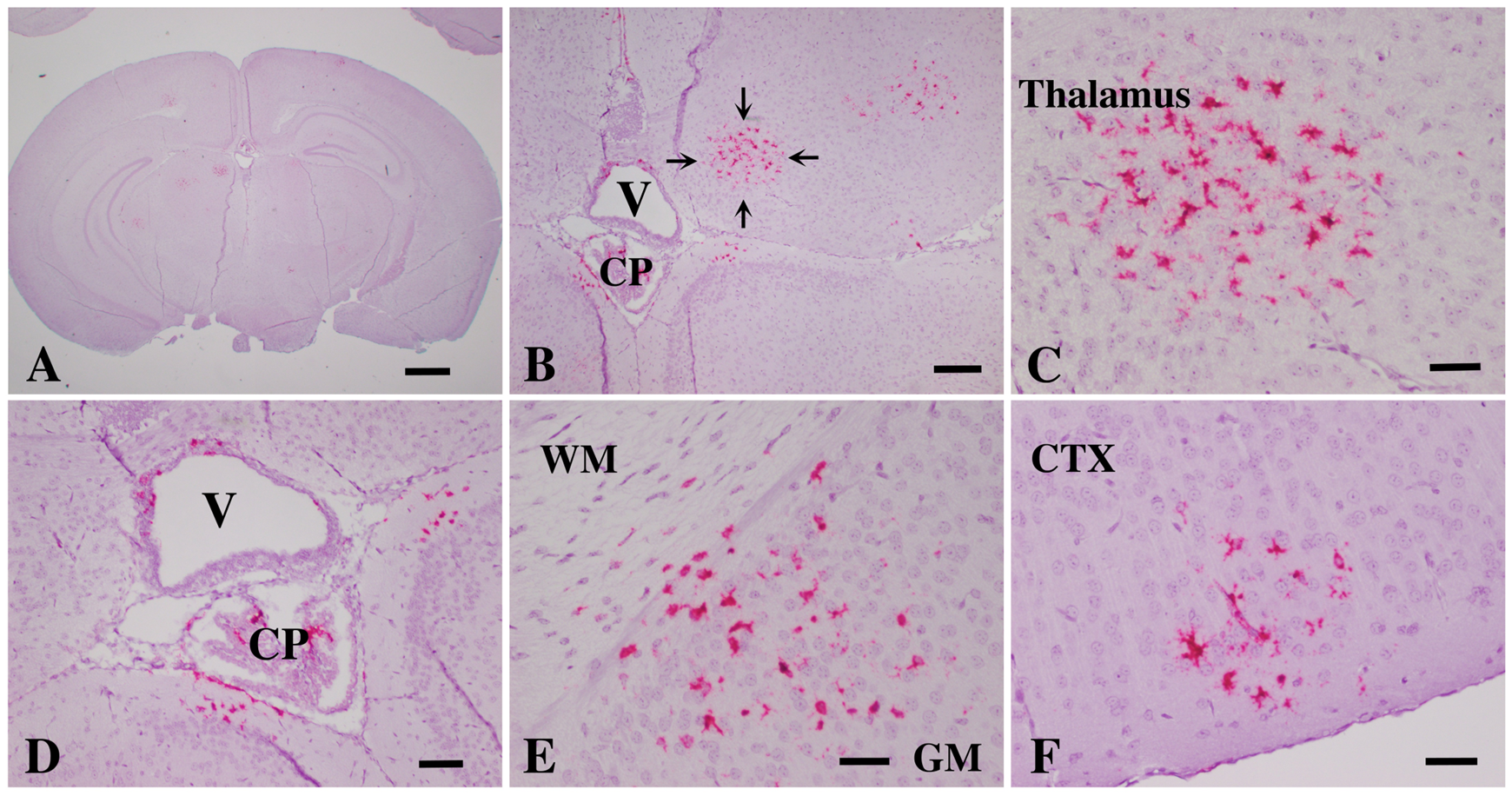
Mouse models for neuroinflammatory and neurodegenerative diseases: the incidence of neurodegenerative diseases, such as Alzheimer's Disease (AD), and AD-related dementia (ADRD), raises in aged populations. Neuroinflammation naturally occurs in aged brains. Preclinical evidence showed that neuroinflammation contributes to neurodegenerative pathogenesis in AD. However, the role of inflammation in AD development is poorly understood. We are analyzing our mouse models to understand how chronic neuroinflammation relates to neurodegenerative diseases and explore strategies to treat these diseases.
Lab Members
- Principal Investigator: Qingde Wang, MD, PhD
Location
NW607 UPMC Montefiore
Publications
Publications from the Wang Lab can be viewed through PubMed.